Plasma Etch
Process
Plasma Etch
- Dry etch processes use gaseous chemical
etchants to react with the materials to be etched and form volatile by
products that will be removed from the substrate surface.
- Plasma generates chemically reactive free
radicals that can significantly increase the chemical reaction rate and
enhance the chemical etch.
- Plasma bombards the wafer surface with ions,
which physically remove material from the surface of the wafer and also
break chemical bonds at the surface. This significantly accelerates the
chemical reaction rate for the etch process.[1]
Plasma
- Plasma is ionized gas with an equal number of
negative and positive charges. It also consists of ions, electrons, and
neutral atoms or molecules.
- The three important collisions in plasma are
ionization, excitation-relaxation, and dissociation.
- These collisions generate and sustain the
plasma, cause the glow of gas discharge, and create the chemically
reactive free radicals that enhance chemical reactions, respectively.
- Mean free path (MFP) is the average distance a
particle can travel before it collides with another particle. Reducing
pressure increases the MFP and ion bombardment energy, and also reduces
ion scattering, helping achieve the vertical etch profile.[3]
Etch Mechanisms
- In the plasma etch process; first the etchants
are introduced into the vacuum chamber.
- After pressure is stabilized, RF power is used
to strike glow discharge plasma. Some of the etchant molecules dissociate
in the plasma from the impact of collisions with electrons, which generate
free radicals.
- The free radicals then diffuse across the
boundary layers, reach the wafer surface, and are adsorbed on the surface.
- With the help of the ion bombardment, these
free radicals react with the surface atoms or molecules very quickly and
form gaseous byproducts.
- The volatile byproducts desorbs from the
surface, diffuse across the boundary layer, get into the convection flow,
and are pumped out from the chamber.
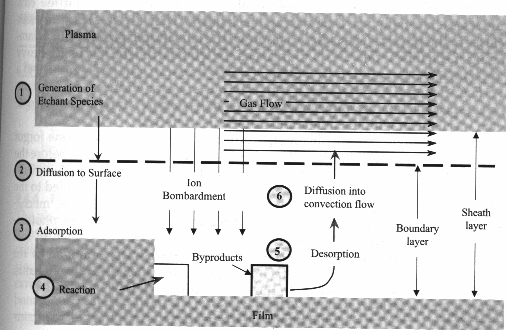
Fig. 6 Plasma etch sequence [3]