Silicon Oxidation Model
Deal and Grove’s model describes
the kinematics of silicon oxidation. The model is generally valid for
temperatures between 700 and 1300°C,
partial pressures between 0.2 and 1 atmosphere, and for oxide thickness between
300 and 20,000°A for oxygen and water
ambients.
Flux is the number of atoms or
molecules crossing a unit area in unit time.
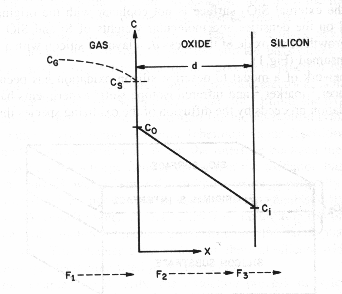
Fig. 2 Basic model for thermal oxidation of
silicon [2]
Basic model for thermal
oxidation of silicon [2]
The oxidizing species:
- Are transported from the bulk of the gas phase
to the gas-oxide interface with flux F1.
- Are transported across the existing oxide
toward the silicon with flux F2.
- React at Si-SiO2 interface with the silicon with flux F3.
Under steady state, all the
fluxes are equal, F1=F2=F3
- Assuming that the flux of the oxidant from the
bulk of the gas phase to the gas-oxide interface is proportional to the
difference between the oxidant concentration in the bulk of gas CG
and the oxidant concentration adjacent to the oxide surface CS,
we get
F1=hG(CG-CS)
-----------------(1)
Where
hG is the gas phase mass-transfer coefficient. [1] [4]
- From Henry’s law, which states that the
concentration of an adsorbed species at the surface of a solid is
proportional to the partial pressure of that species in the gas just above
the solid, we get
C0=HpS
and C*=HpG
Where,
C0
is the equilibrium concentration in the oxide at the outer surface.
C* is the equilibrium bulk concentration in the
oxide.
pS is
the partial pressure in the gas adjacent to the oxide surface.
pG
is partial pressure in the bulk of gas
H is
Henry’s law constant.
- Using Henry’s law along with the ideal gas law
we get,
C0=HpS=HkTCS
where pS=kTCS
and
C*=HpG=HkTCG where pG=kTCG
Substituting,
C0 and C*, into
equation (1), we get the following:
F1=h(C*-C0) where h=hG/HkT
- Oxidation is a non-equilibrium process with
the driving force being the deviation of concentration from equilibrium.
Henry’s law is valid only in the absence of dissociation effects at the
gas-oxide interface. This implies that the species moving through the
oxide are molecular.
- Flux of the oxidizing species across the oxide
follows Fick’s law at any point d in the oxide layer.
F2
= D(C0-Ci)/d0
Where
D is the
diffusion coefficient
Ci
is the oxidizing species concentration in the oxide adjacent to the
oxide-silicon interface.
d0
is the oxide thickness.
- Flux corresponding to the Si-SiO2
interface reaction is proportional to the concentration of oxidizing
species in the oxide adjacent to the oxide-silicon interface, Ci.
F3=kSCi
Where kS
is the rate constant of chemical surface reaction for silicon oxidation.
- Under steady state conditions we have
F1=F2=F3
and solving simultaneous
equations we can find Ci and C0.
- When diffusivity is very small Ci®0 and C0®C*.
This is called diffusion-controlled case. It results from the flux of
oxidant through the oxide (F2) being small compared to the flux
corresponding to the silicon-silicon dioxide interface reaction (F3).
Hence oxidation rate depends on the supply of oxidant to the interface and
not on the reaction at the interface.
- When diffusivity is large, Ci=C0.
This is called reaction-controlled case, because an abundant supply of
oxidant is provided at the silicon-silicon oxide interface and the
oxidation rate is controlled by the reaction rate constant kS
and Ci.
- Combining various equations and assuming that
an oxide may be present initially from a previous processing step, that
is, d0=di at t=0 the following equation can be
generated. [2]
d02
+ A d0 = B(t+t)
Where t represents a shift in the time axis to account for the
presence of the initial oxide layer di.
Also,
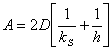
![]()
![]()
N1 is
the number of oxidant molecules incorporated into a unit volume of the oxide
layer.