Introduction
The chemical reactions
describing the thermal oxidation of silicon in oxygen or water vapor are given
by the following equations:
Si(solid)
+ O2 ® SiO2
(solid) -------------------DRY
OXIDATION
Si(solid)
+ 2H2O ® SiO2(solid)
+2H2 -----------WET OXIDATION
For growth of an oxide of
thickness d, a layer of silicon with thickness of 0.44d is consumed. Oxidation
proceeds by the diffusion of the oxidizing species through the oxide to the
Si-SiO2 interface, where the oxidation reaction occurs.
When bare silicon is exposed to
the atmosphere, it reacts almost immediately with oxygen or moisture present in
the air and forms a thin (about 10 to 20°A) layer
of silicon dioxide, called the native oxide. The thickness of the native oxide
is sufficient to stop further oxidation of the silicon at room temperature. [1]
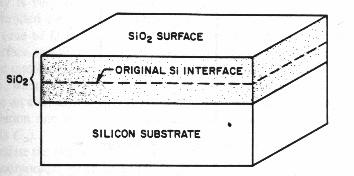
Fig. 1 Silicon Oxidation Process [2]
Uses of
silicon dioxide
- It serves as a mask against implant or
diffusion of dopant into silicon. The thickness of the oxide needed to
mask is about 500°A.
- It provides surface passivation.
- It is used to isolate one device from another.
- It is used as a gate dielectric for MOSFETs.
- It provides electrical isolation of
multi-level metallization systems.
- Silicon oxide is used as a screen oxide for
ion implantation process in order to prevent silicon contamination.[2] [1]