Channeling
Many targets are crystalline in
nature with regular arrangement of atoms. For ions moving in certain
directions, the atom rows or planes line up so that there are long-range open
spaces through which the ions can travel without significant scattering. Ions
are steered down these channels by glancing collisions with the atom rows or
planes thus extending the final ion distribution deeper into the target. This
is known as channeling.
The effect of channeling is to
add a tail to the implanted distribution, which for silicon can be approximated
by an exponential with decay length around 0.1 microns.
We don’t want channeling due to
the following reasons:
- Modeling of channeled ion distributions is
difficult and makes it difficult.
- Also channeled ion profile becomes sensitive
to changes on the order of 1° in
wafer tilt and beam divergence.
Channeling can be eliminated by
the following methods:
- Tilt the wafer by 7° relative to the incoming
beam to give the appearance of a random target, and hence avoid
channeling.
- Ions can be scattered out of channels by
thermal vibrations, lattice defects and foreign atoms.
- It can be reduced due to damage to the crystal
caused by nuclear stopping of unchanneled ions.
- A preceding implant of silicon to amorphize the
lattice structure will also reduce channeling effects.
Electronic interactions will
stop the ion inside the channel. But it should be noted that because the
electron density at the center of a channel is low, the electronic stopping
power for a well-channeled ion would be less than that of an ion traveling
randomly.
Channeling is characterized by a
critical angle, Y1,which
is the maximum angle between ion and the channel for a glancing collision to
occur.
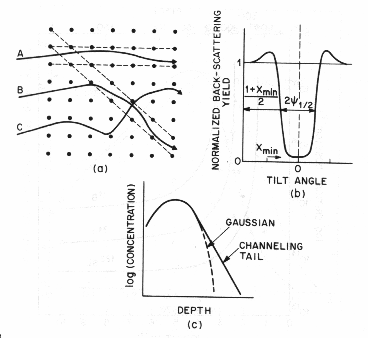
Fig. 7 Schematic views of
channeling (a) Ion paths through a cubic lattice, showing channeled and nonchanneled cases. (b)
Back-scattering yield around a channeling direction. (c) the effect of
channeling is to add a tail to the atom distribution.[2]