Solid-solubility
limit
At a given temperature, there is an upper limit to the
amount of an impurity, which can be absorbed by silicon. This quantity is
called the solid-solubility limit for
the impurity and is indicated by solid lines in the figure below for boron,
phosphorus, antimony, and arsenic at normal diffusion temperature [1]. For
example, the solid-solubility limit for boron is approximately 3.3X1020/cm3
at 1100°C and 1.2X1021/cm3
for phosphorus at the same temperature [1]. Surface-concentrations achieved
through solid-solubility limited diffusions are quiet high and are useful for
emitter and collector regions in bipolar transistors and also for, source and
drain regions in MOSFETs.[1]
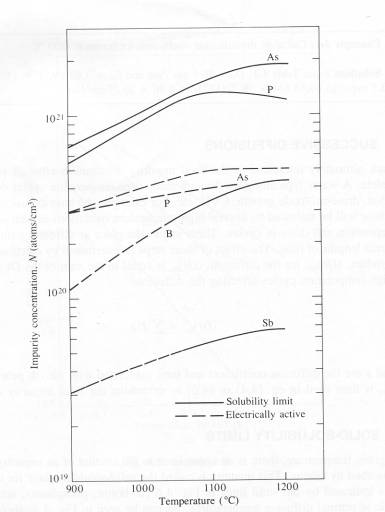
Solid solubility and electrically active impurity-concentration
limits in silicon[1].