Point Defects
Point
defects take several forms as shown in figure. Any non-silicon atom
incorporated into the lattice at either a substitutional (i.e., replacing a
host silicon atom) or interstitial (i.e., between silicon atoms) site is
considered a point defect.
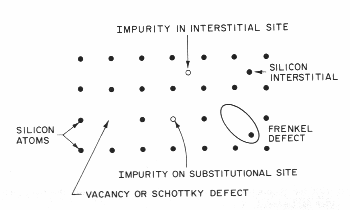
Fig.
5 the location and types of point defects in a simple lattice.[2]
Missing
atoms create a vacancy in the lattice called a "Schottky defect,"
which is also considered a point defect. A silicon atom in an interstitial
lattice site with an associated vacancy is called a "Frenkel defect."
Vacancies
and interstitials have equilibrium concentrations that depend on temperature.
From thermodynamic principles the following relation gives the concentration of
the point defect as a function of temperature. [2]
Nd = A exp(-Ea/kT)
Where
Nd is the concentration of the point
defect
A is a constant
Ea is the activation energy
T is the absolute temperature
k is Boltzmann's constant
Point defects are important in the kinetics of
diffusion and oxidation. The diffusion of many impurities depends on the
vacancy concentration, as does the oxidation rate of silicon.