Five Main
Cubic Structures
1. Simple
cubic crystal
Each
corner of the cubic lattice is occupied by an atom, which is shared by eight
neighboring unit cells. It should be noted that only 1/8 of each corner atom is
actually inside the cell (Fig. 2(a) and 2(b)).
2. Body-centered
cubic crystal
In
bcc crystal in addition to the corner atoms, an atom is also located at the
center of the cube (Fig. 2(c)).
3. Face-centered
cubic (fcc) crystal
It
contains one atom at each of the six cubic faces in addition to the eight
corner atoms. Note however that only one-half of each atom actually lies inside
the fcc unit cell (Fig. 2(d)).
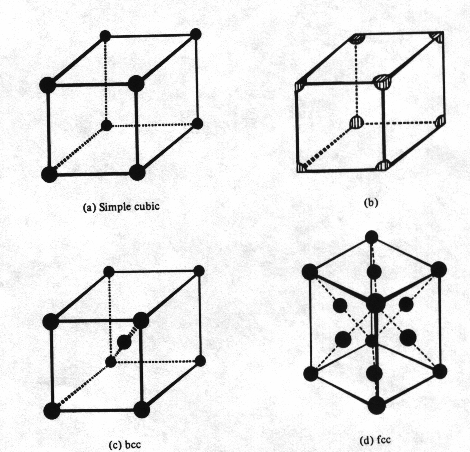
Fig.
2 Simple three-dimensional unit cells (a) Simple cubic unit cell. (b)
Pedantically correct simple cubic unit cell including only the fractional
portion of each corner atom actually within the cell cube. (c) Body centered
cubic unit cell. (d) Face centered cubic unit cell.[1]
4. Diamond
structure
A
diamond unit consists of two interpenetrating fcc sublattices with one
displaced from the other by one-quarter of the distance along a diagonal of the
cube. Both Silicon and Germanium exhibit this structure. The corner and face
atoms of the unit cell can be viewed as belonging to one fcc lattice, while the
atoms totally contained within the cell belong to the second fcc lattice. The
second lattice is displaced one-quarter of a body diagonal along a body
diagonal direction relative to the first fcc lattice (Fig. 3(a)).
5. Zinc
blende structure
It
is formed when the Gallium atoms are placed on one lattice and Arsenic atoms on
the other fcc lattice in a diamond structure, example is GaAs(Fig. 3(b)).
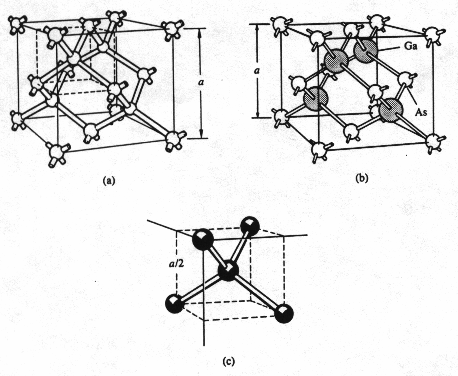
Fig.
3 (a) Diamond lattice unit cell. (b) Zincblende lattice unit cell. (c) Diamond
lattice emphasizing the four-nearest neighbor bonding within the structure. [1]